Green energy
Waste to hydrogen
Feb 23 2021
Author credit - Stephen B. Harrison, Managing Director, sbh4 consulting
Thermochemical processes such as gasification, reforming and pyrolysis can convert hydrocarbon-rich solid wastes to hydrogen. These thermochemical processes yield syngas which can be further processed to hydrogen, a clean, emission-free energy vector. The thermochemical processes that yield syngas differ from waste incineration, which takes place in an excess of oxygen to generate steam, carbon dioxide and ash.
Thermochemical processing of waste does produce some carbon dioxide, but the International Panel on Climate Change (IPCC) regards processing of the biogenic fraction of waste as carbon dioxide neutral. Animal carcasses, scrap wood, waste vegetable oils and post-consumer wastepaper meet this definition.
Integrated pyrolysis, reforming and gasification have a significant environmental advantage over incineration: the oxygen-deficient atmosphere prevents the formation dioxins and furans which are highly toxic pollutants. Organic materials ranging from municipal solid waste, biomass, sewage, manufacturing waste, plastic waste, hospital waste, agricultural and slaughterhouse waste are suitable feedstocks.
After removal of recyclables, the waste is shredded and fed into the first thermochemical processing reactor stage which is pyrolysis. Pyrolysis takes place at around 750 °C in the absence of air. The reactor can be externally heated, or the heat may be applied to a heat carrier medium in a fluidised bed reactor, or moving bed reactor. The feedstock is thermally decomposed into gaseous, solid, and liquid components.
As the gases leave the pyrolysis reactor, steam is added to enable reforming and cracking reactions. These generate hydrogen and break larger molecules such as tars to form short-chain hydrocarbons. Reforming generally takes place at around 920 °C.
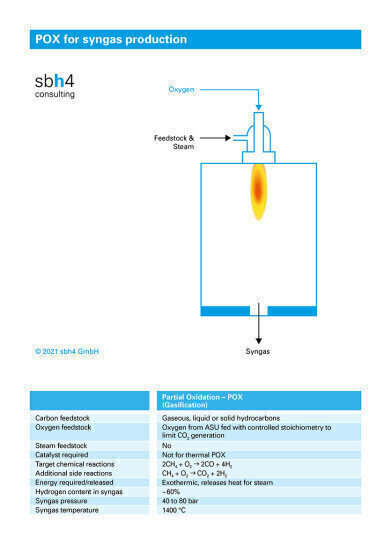
As a third thermochemical processing stage, a partial oxidation reactor (POX) can be used. The POX reactor is fed with pure oxygen, rather than air, to avoid NOx generation. NOx causes air pollution and NOx generation within the reactor would require its removal using SCR or SNCR techniques. In the POX reactor a controlled amount of oxygen is added – less than the amount required for complete combustion. This ensures that carbon monoxide is produced in preference to carbon dioxide.
Syngas leaving the exothermic POX reactor is at a very high temperature. Recovery of heat from the syngas with a radiant/convective heat exchanger or direct quench improves the overall process efficiency. The high-grade heat can be used to produce steam for power generation or process heating.
Typical syngas clean-up and conditioning processes include cyclones and filters for particulates removal followed by wet gas scrubbing to remove fine particulates, ammonia, and chlorides. Solid absorbents such as activated carbon may also be used to remove mercury and other trace heavy metals.
Fischer-Tropsch conversion of the clean syngas to produce methanol or liquid fuels is a possible next step in the process. Or, if hydrogen is the target, the syngas is fed to water gas shift (WGS) reactors which progressively increase the ratio of hydrogen to carbon monoxide (CO) in the syngas.
To maximise the hydrogen yield, the WGS reaction can be carried out in two stages. The first is a high-temperature shift (HTS) reactor which operates at 300–450 °C. Then comes the low-temperature shift (LTS) reactor which operates at 180–230 °C. Steam injection can be used to optimise the reaction conditions and favour hydrogen production.
Finally, pressure swing adsorption (PSA) is used to separate hydrogen from the syngas. CO and some hydrogen exit the PSA as ‘tail-gas’ which can be burned with some additional natural gas in a mixed-fuel burner system to create the energy that is required for the endothermic pyrolysis and reforming reactions.
The PSA equipment generates hydrogen at a purity between 98 and 99.999%. At the lower end of that purity range, hydrogen can be used for industrial and chemical processes such as desulphurisation of crude oil to make clean fuels. It could also be used for direct reduction of iron to replace coke in a blast furnace. At higher purities close to 99.999%, the hydrogen can be fed to fuel cells for mobility and transportation applications.
Alternatively, Hydrogen can be fed to a Haber Bosch reactor to produce ammonia which is also an energy vector. Ammonia and hydrogen emit zero carbon dioxide when they are burned. So, they can help with the transition to green energy, decarbonisation, and the battle against climate change. Conversion of hydrogen to ammonia is sometimes advantageous for long distance shipping to leverage existing ammonia distribution infrastructure and tankers.
Events
Jun 17 2025 Guangzhou, China
Singapore International Water Week Spotlight 2025
Jun 23 2025 Singapore
Jun 25 2025 Sao Paulo, Brasil
Jul 02 2025 Bangkok, Thailand
Jul 02 2025 Bangkok, Thailand