Air clean up
Low GWP ammonia refrigerant gas for CCUS
Nov 13 2023
Author credit – Stephen B. Harrison, sbh4 consulting
The full supply chain to connect CO2 sources to users could be called “CCTUS” or CO2 capture, transportation and utilisation or storage. The mid-stream CO2 transportation segment is either done using supercritical CO2 through compression, or with liquid CO2.
CO2 utilisation to produce e-fuels such as methanol and synthetic aviation fuel (eSAF) is destined to ramp up during this decade. This will pull for a flexible liquid CO2 distribution network enabled by new CO2 transporter ships and rail tankers. Efficient CO2 liquefaction will be at the heart of this value chain.
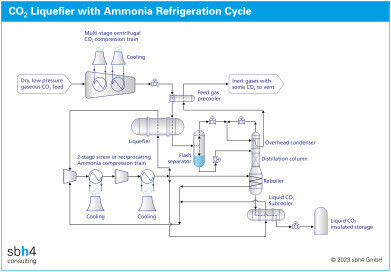
CO2 liquefaction can be achieved using chillers that are charged with F-Gases or ammonia. Use of ammonia as a refrigerant gas is widely accepted as the most efficient way to liquefy CO2 at scale. Ammonia also eliminates the Global Warming Potential (GWP) risk of inadvertent F-Gas emissions. Since the motivation to capture and utilise CO2 is to avoid greenhouse gas emissions, the use of a GWP-free refrigerant gas is aligned with the overall concept of CCUS.
Ammonia be used in most parts of the world for CO2 liquefiers. In extremely warm locations, where peak summer temperature may rise to around 50 °C, the system must be designed for a slightly higher operating pressure.
In the CO2 liquefier, the CO2 gas is compressed to around 20 bar to enable its liquefaction at a moderate temperature. There is a trade-off between the size and cost of the CO2 compressor and the size of the refrigerant gas compressor and its temperature of operation.
Normally the ammonia compressor suction pressure is just above atmospheric pressure at around 0.1 psi(g). This avoids the risk of air ingress into the system. Allowing for the pressure drop of gaseous ammonia between the CO2 liquefier and the ammonia compressor suction, the corresponding liquid ammonia temperature in the CO2 liquefier will be close to -33 °C.
Like ammonia, R12 could achieve -29 °C. Other F-Gases can achieve lower temperatures (R22 down to -40C, R404A down to -45 C and R410A down to -51 C), but the compressor must operate at a higher pressure and therefore consumes more power. A larger and more expensive compressor would also be required.
Of the above F-Gases gases, R404A has a GWP of 3,920. R410A has a lower GWP of 2,088. R22 is slightly less at 1,810, however, unlike R404A and R410 it does not have zero Ozone Depletion Potential (ODP). Due to the presence of chlorine in the R22 molecule, its ODP is 0.05.
The GWP of ammonia is zero. It also has zero ODP. There is no other refrigerant gas which would be suitable for CO2 liquefaction that has these superior environmental credentials.
Ammonia has a higher latent heat of evaporation compared to F-Gases such as R12 and R404A. The implication is that only 1/7th of the refrigerant gas inventory is required. Compared to these F-Gas alternatives, ammonia has a lower condensation pressure and temperature which means a higher co-efficient of performance (CoP) and less electrical power consumption is required to achieve the same cooling effect.
Ammonia allows for a flooded condenser design, which generates a higher efficiency for heat transfer than gas-to-gas heat exchangers that would be required when using other refrigerant gases. The flooded system also allows for smaller pipe sizes, which helps to keep capital costs low.
Heat is removed from the ammonia through heat exchange with cooling water through an evaporative condenser. Ammonia flows through the tubes of the condenser, and these are immersed in the cooling water to cool the ammonia as close as possible down to the wet bulb temperature of water.
Events
Mar 31 2025 Hannover, Germany
Apr 02 2025 Saigon, Vietnam
Apr 08 2025 Targi Kielce, Poland
Apr 08 2025 Bahrain
Apr 10 2025 Beijing, China