Wastewater treatment
Scientists at the University of Manchester develop ‘molecular trap’ to tackle water pollution
Feb 05 2025
Researchers from The University of Manchester have created a groundbreaking material designed to help reduce water pollution caused by harmful chemicals—such as traces of medications and hygiene products—that often end up in rivers and lakes.
Water pollution is becoming an increasingly urgent environmental issue. Everyday items like medications, personal care products, and cosmetics leave behind chemical residues that don’t fully break down after use. These pollutants make their way into water systems, disrupting ecosystems and posing threats to plants, animals, and human health.
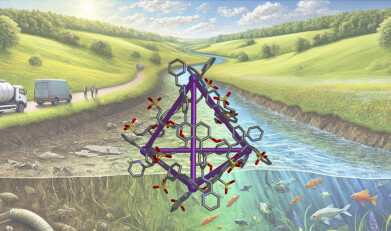
The research, published in Cell Reports Physical Science, introduces a novel method using metal-organic cages (MOCs)—tiny, cage-like structures that act as traps for harmful molecules found in water. While MOCs have been studied before for their ability to capture gases and chemicals, their application in water systems, where they face different challenges than in solvents, marks a significant breakthrough.
Jack Wright, a researcher at The University of Manchester and PhD candidate, explained: “The ability to apply MOCs to water systems is an exciting step forward. While MOCs have been proven effective in capturing unwanted substances, this is the first time they've been shown to work in real-world water environments.”
Water pollution, particularly from difficult-to-remove chemicals, is a global crisis. Wright adds, “This new MOC technology could become an invaluable tool to help clean up water systems, preventing pollutants from entering ecosystems—especially in urban and industrial areas where wastewater discharge is most common.”
The MOCs are made up of metal ions linked by organic molecules, forming hollow, pyramid-like structures. These cavities trap specific molecules, such as pollutants or gases. By incorporating sulfonates into the MOCs, the researchers made them water-compatible, allowing them to function in natural water systems like rivers or wastewater.
Using a natural phenomenon known as hydrophobic binding, the MOCs cause contaminants to cling to the inside of the cage, rather than remaining in the water. This selective capture allows the material to effectively trap pollutants in challenging water conditions.
Dr. Imogen Riddell, PhD supervisor and researcher at The University of Manchester, emphasised the flexibility of the approach: “One of the key strengths of this method is its adaptability. We’ve developed a system that could be used to create MOCs with different sizes and properties. This opens up a wide range of future possibilities, including cleaning up various pollutants, developing green catalysts, and even advancing drug delivery techniques.”
The team now plans to expand the capabilities of their water-soluble cages to capture a broader range of contaminants. They are also exploring ways to recycle the MOCs, paving the way for their sustainable use in water purification efforts.
Events
Mar 18 2025 Expo Santa Fe, Mexico
Mar 18 2025 Moscow, Russia
Mar 19 2025 Manila, Philippines
Mar 20 2025 Guangzhou, China
Mar 24 2025 National Harbour, MD, USA